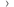A trial looking at AMG 319 for head and neck cancer
Cancer type:
Status:
Phase:
This trial looked at AMG 319 for people with  of the mouth or throat. It was for people who were due to have surgery to remove their cancer.
of the mouth or throat. It was for people who were due to have surgery to remove their cancer.
The trial was supported by Cancer Research UK. It was open for people to join between 2015 and 2018. The team reported results between 2019 and 2023.
More about this trial
AMG 319 is a type of targeted cancer treatment. Doctors wanted to find out whether it can help the  find and kill cancer cells.
find and kill cancer cells.
They wanted to see how well it worked for people with  . Those taking part had cancer of the:
. Those taking part had cancer of the:
They were put into a treatment group at random. Some people had AMG 319. And some people had a dummy drug (placebo). They had treatment for 3 to 4 weeks, and then an operation to remove their cancer.
The main aims of the trial were to find out:
- how AMG 319 affects the immune system
- more about the side effects of AMG 319
- how AMG 319 works in the body
Summary of results
The trial showed that AMG 319 had an effect on some parts of the immune system.
Trial design
A total of 32 people joined this trial. The trial team closed the trial earlier than planned, so fewer people joined than they’d hoped.
The people taking part were put into a treatment group at random. There were:
- 23 people in the group who had AMG 319
- 9 people in the group who had the dummy drug (placebo)
Of these, 30 people went on to have treatment:
- 21 people had at least one dose of AMG 319
- 9 people had at least one dose of the placebo
The research team measured levels of immune system cells before and after treatment in both the cancer and in blood samples.
This included white blood cells called CD8+ cells. These cells can help the immune system recognise and kill cancer cells. The team hoped AMG 319 would increase the number of CD8+ cells in the cancer.
They also measured FoxP3+ cells in the cancer. These cells help regulate the immune system and lower the immune response. This can stop the immune system being able to recognise and kill cancer cells. The team hoped AMG 319 would lower the number of FoxP3+ cells.
The team also looked at the effect AMG 319 had on immune cells in the blood. One of the cells they looked at were Treg cells. They are a type of  which help regulate our immune response.
which help regulate our immune response.
Results
The results from looking at the cancer cells showed that AMG 319:
- didn’t increase the number of CD8+ cells
- decreased the number of FoxP3+ cells
The results from looking at the blood samples showed that AMG 319 increased the number of Treg cells.
The trial team looked at how well AMG 319 worked as a treatment. They found that the cancer:
- went away in 1 person
- shrunk to less than half the size in 2 people
- stayed the same in 16 people
They were not able to assess this for 11 people who had treatment.
Side effects
Most people (91%) had at least one side effect caused by AMG 319. Many of these were not serious. But more than 4 out of 10 people (43%) had at least one side effect that was more serious.
In total, 10 people decided to stop treatment because of the side effects they were having.
Non serious side effects
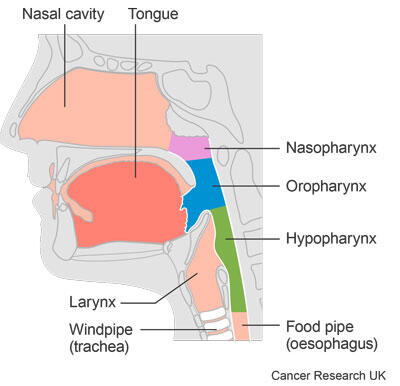
The most common non serious side effects were:
- 13 people (62%) had a rash
- 12 people (57%) had diarrhoea
- 9 people (43%) had flu-like symptoms
- 8 people (38%) had a high temperature (pyrexia)
- 8 people (38%) had an increase in a liver protein called ALT
- 6 people (29%) felt sick (nausea)
- 6 people (29%) were sick (vomited)
- 5 people (24%) had a drop in white blood cells called lymphocytes
- 5 people (24%) had a change in taste (dysgeusia)
Serious side effects
Researchers can class a side effect as serious for a number of reasons, including if:
- the person has to go to hospital because of it
- it is particularly important for the specific treatment in the trial
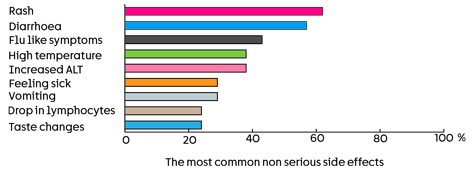
The most common serious side effects were:
- 5 people (24%) had a high temperature (pyrexia)
- 4 people (19%) had diarrhoea
- 3 people (14%) had a rash
- 2 people (10%) had inflammation of the bowel (colitis)
- 2 people (10%) were sick (vomited)
Conclusion
The research team concluded that the doses of AMG 319 in this trial caused some changes in the immune cells around the cancer. These helped the immune system attack the cancer cells.
AMG 319 also increased the immune cells in the blood. But this caused too many severe side effects.
Overall, the side effects people had outweighed the possible benefits of treatment in this trial. The team suggest that having different doses of AMG 319, or having treatment at different times, may help reduce the side effects.
Future plans
When this summary was written there were no plans in place to look at AMG 319 in other trials.
More detailed information
There is more information about this research in the reference below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
Intermittent PI3Kδ inhibition sustains anti-tumour immunity and curbs irAEs
S Eschweiler and others
Nature, 2022. Volume 605, pages 741–746.
Where this information comes from
We have based this summary on the information in the article above, and other information provided by the trial team. The information in the article above has been reviewed by independent specialists ( ) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link above is active and the article is free and available to view.
) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link above is active and the article is free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Christian Ottensmeier
Supported by
Cancer Research UK - regulatory@cancer.org.uk ( )
)
Amgen
Experimental Cancer Medicine Centre (ECMC)
NIHR Clinical Research Network: Cancer
The research team would like to thank all the patients who took part in this trial. By doing this, you’ve helped them learn more about cancer and how to treat it.
Other information
The CRUK trial number is CRUKD/15/004.
There is more information about this trial on the clinicaltrials.gov website (NCT02540928):
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040