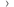A trial looking at MOv18 IgE for advanced solid tumours
Cancer type:
Status:
Phase:
This trial looked at a drug called MOv18 IgE for people with advanced cancer. It was for people who’d had all the standard treatments available to them.
The trial was supported by Cancer Research UK. It was open for people to join between 2016 and 2021. The team analysed the results in 2022.
More about this trial
MOv18 IgE is a type of targeted cancer treatment called a monoclonal antibody.
Monoclonal antibodies work by finding certain proteins on the surface of cells. They help the immune system to find and attack these cells.
Some cancer cells have a protein called folate receptor alpha. MOv18 IgE finds these cancer cells by binding to this protein.
When this trial was done, researchers had already been looking at how MOv18 IgE works in the laboratory. But this study was the first time it had been given to people.
The main aims of this trial were to find out:
- more about the side effects
- how the side effects were related to the dose people had
- the best dose of MOv18 IgE to use in future trials
- if it may be a useful treatment for people with advanced cancer
- what happens to MOv18 IgE in the body
Summary of results
The trial team found that the doses of MOv18 IgE they used didn’t cause too many serious side effects They weren’t able to find the best dose to use.
Trial design
This trial was for people with advanced cancer who didn’t have any standard treatments available to them. Everyone in the study had MOv18 IgE, but they had different doses.
The first few people had the lowest dose. As they didn’t have any major side effects, the next few people had a higher dose, and so on. This is called dose escalation.
The research team looked at 8 different doses of MOv18 IgE. Between 1 and 6 people had each dose.
Results
A total of 26 people joined this trial. They all had cancer which lab tests showed had the folate receptor alpha protein. They were all women between 47 and 79 years old.
The people taking part had a number of different cancers:
- 20 people had ovarian cancer
- 3 people had fallopian tube cancer
- 2 people had womb (endometrial) cancer
- 1 person had high grade serous cancer
They’d all had treatment for their cancer, but it had continued to grow or had spread to another part of the body (but not to the brain).
MOv18 IgE is a treatment you have through a drip into a vein. People taking part had treatment once a week for up to 6 weeks. If their cancer hadn’t grown after 6 weeks, they could have more MOv18 IgE. They could have 3 more doses over 6 weeks.
Pre treatment checks
Everyone taking part had a very small dose of MOv18 IgE put into the skin of their forearm, before they had treatment. This was to check if they had an allergic reaction. People who did have a reaction didn’t go on to have treatment as part of the trial.
The research team injected a small amount of MOv18 IgE just under the skin for the first 7 people who took part. This is called intradermal testing. Five people had a positive reaction. They didn’t have any more MOv18 IgE.
Everyone else had a small amount of MOv18 IgE put onto the skin, which was gently pricked. This is called a skin prick test. No one had a positive skin prick test.
Side effects
Nearly 9 out of 10 people (89%) who took part had at least one side effect caused by treatment. Many of these were mild or didn’t last long. But some were more serious. Four people stopped treatment because of side effects they were having.
Non serious side effects
The most common non serious side effects were:
- 14 people (54%) had an itchy rash (urticaria)
- 11 people (42%) had itching (pruritis)
- 7 people (27%) had reddening of the skin (erythema)
- 5 people (19%) had tiredness
- 4 people (15%) had a red bumpy rash (maculopapular rash)
- 3 people (12%) had a headache
Serious side effects
Researchers can class a side effect as serious for a number of reasons, including if:
- the person has to go to hospital because of it
- it is particularly important for the specific treatment in the trial
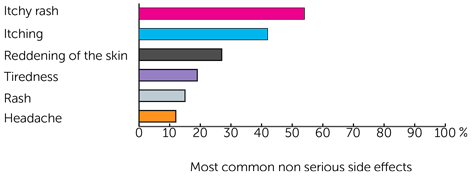
About 3 out of 10 people (31%) had at least one side effect that was classed as serious. Some people had more than one.
One person had an allergic reaction. After this, people could have drugs to help prevent a reaction before they had MOV18 IgE.
The other serious side effects were:
- 5 people (19%) had a positive skin (intradermal) test
- 2 people (8%) had an itchy rash (urticaria)
- 1 person (4%) had low blood pressure (hypotension)
Finding the best dose to use in future trials
The people who had the lowest dose of MOv18 IgE didn’t have any major side effects. So the next few people had a higher dose. And so on for 8 different doses.
Even the people who had the highest dose didn’t have side effects that would suggest the dose of MOv18 IgE was too high. The side effects people had didn’t seem to be affected by the dose.
More trials are needed to find the best dose.
How well treatment worked
The research team looked at how well MOv18 IgE had worked as a treatment for cancer in 20 people who took part. But it’s hard to draw any firm conclusions because of the small number of people.
They found that the cancer had:
- stayed the same in 11 people (55%)
- continued to grow in 7 people (35%)
They were not able to get results for 2 people (10%).
One person had a drop in a  called CA-125. And scans showed that their cancer hadn’t grown. This could have been because the treatment was helping.
called CA-125. And scans showed that their cancer hadn’t grown. This could have been because the treatment was helping.
What happened to MOv18 IgE in the body
The research team took blood samples at various points after treatment to check the levels of MOv18 IgE. They found there the levels of MOv18 IgE in the blood went up with the higher dose levels.
Conclusion
The research team concluded that the doses of MOv18 IgE people had didn’t cause too many serious side effects. They suggest that other trials are done to look at higher doses, and find the best dose to use.
They also learnt more about what happens to MOv18 IgE in the body.
Other trials of MOv18 IgE in the future may show different results.
Future plans
The research team have learned more about MOv18 IgE and cancer treatment from doing this trial. At the time of writing this summary, a company called Epsilogen are planning a trial to look at MOv18 IgE as a cancer treatment.
Where this information comes from
We have based this summary on information from the research team. As far as we are aware, the information they sent us has not been reviewed independently ( ) or published in a medical journal yet. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
) or published in a medical journal yet. The figures we quote above were provided by the research team. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr James Spicer
Supported by
Cancer Research UK (Centre for Drug Development) -  , regulatory@cancer.org.uk
, regulatory@cancer.org.uk
NIHR Clinical Research Network: Cancer
The research team would like to thank all the patients who took part in this trial. By doing this, you’ve helped the research team learn more about cancer and how to treat it.
Other information
This is Cancer Research UK trial number CRUKD/14/001.
There is more information about this trial on the clinicaltrials.gov website (NCT02546921):
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040